CMI scientists at Idaho National Laboratory conducted this research.
Achievement:
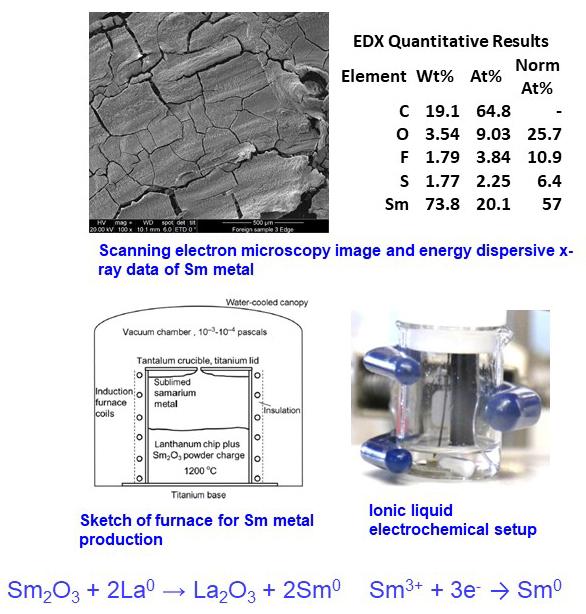
Electrochemically produced samarium (Sm) metal at room temperature in an ionic liquid
Significance and Impact:
- Industrially, Sm metal cannot be produced by electrowinning because of its high vapor pressure
- However, by using ionic liquids, Sm metal can be produced for use in Sm-Co permanent magnets
Details and Next Steps:
- 50 mM Sm in N- methyl-N'-propylpiperidinium bis(trifluoromethylsulfonyl)imide (MPPIP Tf2N)
- Deposition rate of 1.99 mg per cm2/h, with a 72% current efficiency
