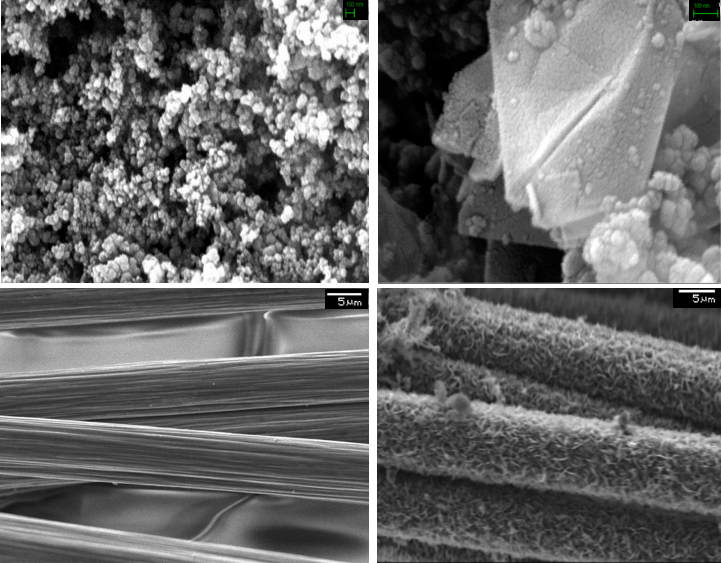
In both cases, the sample undergoes graphitization as evidenced by the presence of flakes that are characteristic of graphitic structure. Carbon fiber was graphitized while maintaining its fibrous bulk morphology.
CMI scientists at Oak Ridge National Laboratory conducted this research.
Achievement:
A novel molten-salt technology for low-temperature graphitization of amorphous carbonaceous materials has been developed.
Impact:
Reducing the temperature from 3100 °C to 800 °C decreases the cost and time to graphitize amorphous carbons. TEA indicates:
- >90% energy savings vs high temperature.
- 50% reduction in unit production costs.
- Synthesis time drops from 3 weeks to 3 hours.
A new domestic supply chain is expected.
Approach:
Cathodic polarization of hard carbon sources in molten CaCl2 and MgCl2 salts was used in the transformation to graphite. Polyacrylonitrile-based carbon fiber, hard carbon, and bio-derived hard carbon graphitized.
