CMI researchers at Case Western Reserve University conducted the research for this highlight
Innovation
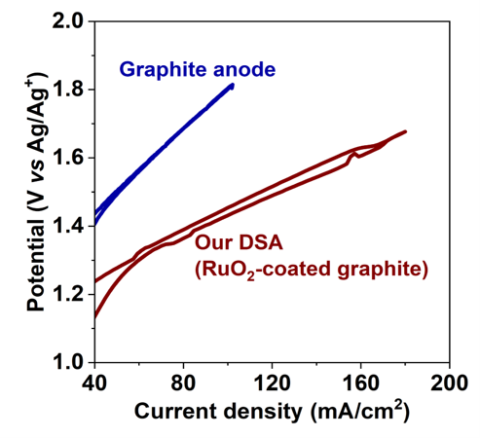
Dimensionally stable anode (DSA) for chlorine evolution in chloride fused salts.
Achievement
Novel DSA fabricated via electrodeposition of RuO2 coatings onto graphitic substrates that are electrocatalytic to Cl2 evolution in molten salt systems. Retains catalytic effect after long use times.
Significance and Impact
Catalytic, stable surface with low reagent cost, low energy consumption, and short production time. Improves economics of electrowinning neodymium from chloride melt, an environmentally-benign production route.
Hub Goal Addressed
Sustainable production of rare earth metals.