CMI researchers at Oak Ridge National Laboratory and Ames Laboratory conducted the research for this CMI highlight
Achievement
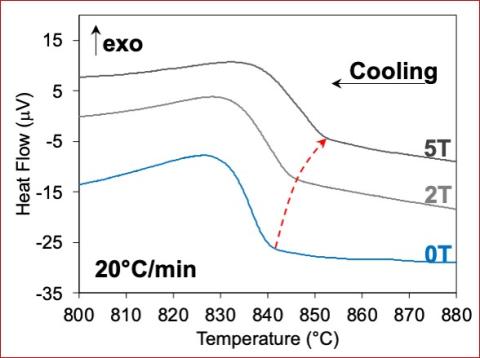
A fully functional calorimeter which operates at high heating rates via induction heating and with static magnetic fields has been granted a patent.
Significance and impact
- The invention enables measurement of transformation temperatures in bulk materials for magnetic fields (0-9 T), at high temperatures (up to 1750 C), and at high heating rates (>1000 C/min)
- Streamlines experimental design to optimize heat treatments in magnetic and other materials
- Compatible with existing commercial processing technology
Next steps
Publish existing data and continue developing technology to achieve quantitative heat flow measurements.
