CMI researchers at Idaho National Laboratory conducted the research for this highlight
Achievement
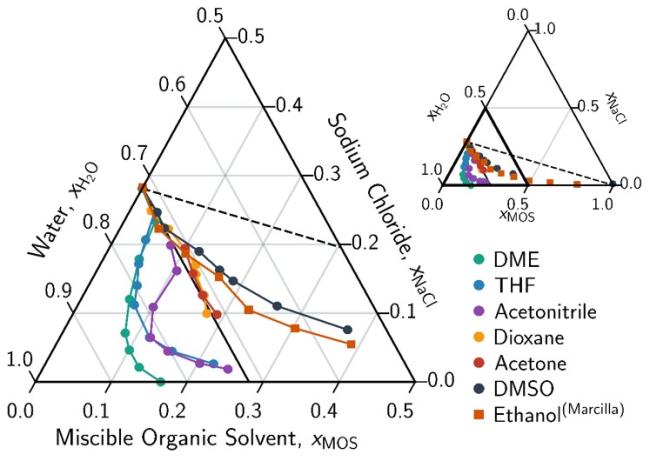
A new limiting law governing solvent driven fractional crystallization has been identified based on solvent driven dewatering and solute precipitation.
Significance and impact
- One-to-one molar displacement of salt by miscible organic solvents (MOS) predicts the SLE phase boundary at low MOS concentrations for all MOS.
- Dimethyl ether (DME) driven water selective extraction (solution concentration and zero liquid discharge) and fractional-crystallization (softening and separations) model as a cost-effective approach.
- Provides a foundation for applying solvent driven processes to critical materials processing.
Details and next steps
Conduct similar studies with CM to validate theoretical model and provide proof of concept for CM primary and secondary processing.
