CMI researchers at Arizona State University conducted the research for this highlight
Innovation
Investigated energetic properties of rare earth (RE) doped CaF2 (Ca1−xRExF2+x) by means of experimental methods for the first time.
Achievement
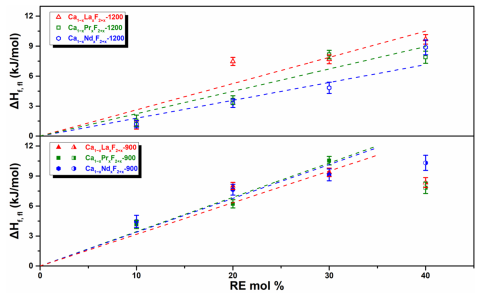
- Determined formation enthalpies of Ca1−xRExF2+x (RE = La, Pr, and Nd, x = 0−0.4) by high temperature oxide melt solution calorimetry.
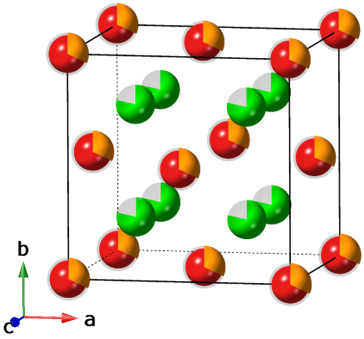
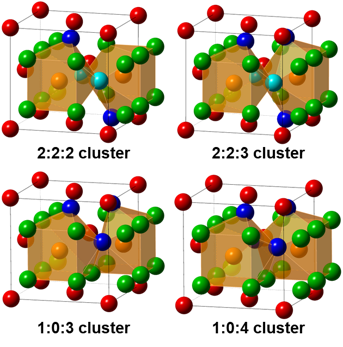
- Characterized crystal structure and defect clusters of Ca1−xRExF2+x solid solutions by powder X-ray diffraction (PXRD) and solid state nuclear magnetic resonance (SS NMR).
Significance and Impact
- CaF2 doped with light RE elements has endothermic formation enthalpies, is entropy-stabilized at solid-state reaction temperatures.
- Defect clusters can energetically stabilize fluorite structure doped with trivalent RE ions, which are affected by RE ionic size, dopant concentration, and annealing temperature.
Hub Target Addressed
Provide fundamental understanding of defect clusters in substitutional solid solutions containing anion interstitials; guide design, synthesis, and functionalization of these optical materials.
Yang S, Anderko A, Riman RE, Navrotsky A, Energetics of Anion Excess Ca1−xRExF2+x (RE = La, Pr, and Nd) Solid Solutions. 2022, to be submitted.
