CMI researchers at Oak Ridge National Laboratory and Ames Laboratory conducted the activity for this highlight
Innovation
Develop chelators that can selectively recognize and leach large vs small rare earth elements (REEs) from mineral sources.
Achievement
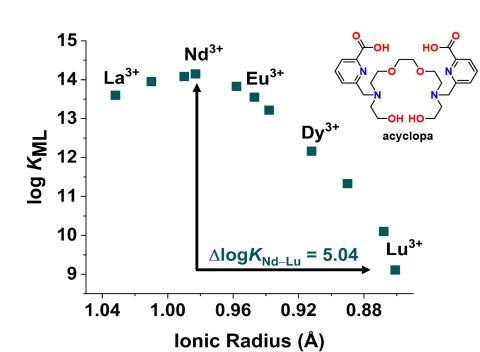
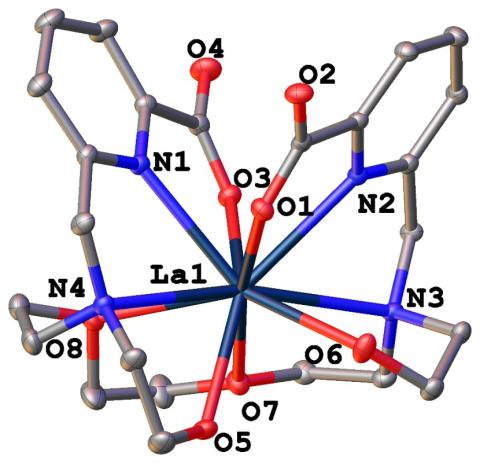
Designed and synthesized “acyclopa,” a novel acyclic chelator that possesses 5 orders of magnitude greater binding affinity for light, large REEs over heavy, small REEs.
Significance and Impact
- Opens the door to developing highly selective leaching agents, potentially enabling process intensification of RE recovery from ores.
- RE-ion selectivity pattern is synergistic with the solubility bias of RE hydroxide, RE(OH)3, in which large RE(OH)3 is more soluble than small RE(OH)3, thereby amplifying resultant separation selectivity.
- Next step: evaluate the efficiency of acyclopa as a leaching agent to selectively dissolve large REEs from caustically cracked monazite, RE(OH)3.
Hub Target Addressed
Unlocking unconventional resources; highly selective separation from complex sources; intra-REE separation.