CMI researchers from Oak Ridge National Laboratory conducted the activity for this highlight
Innovation
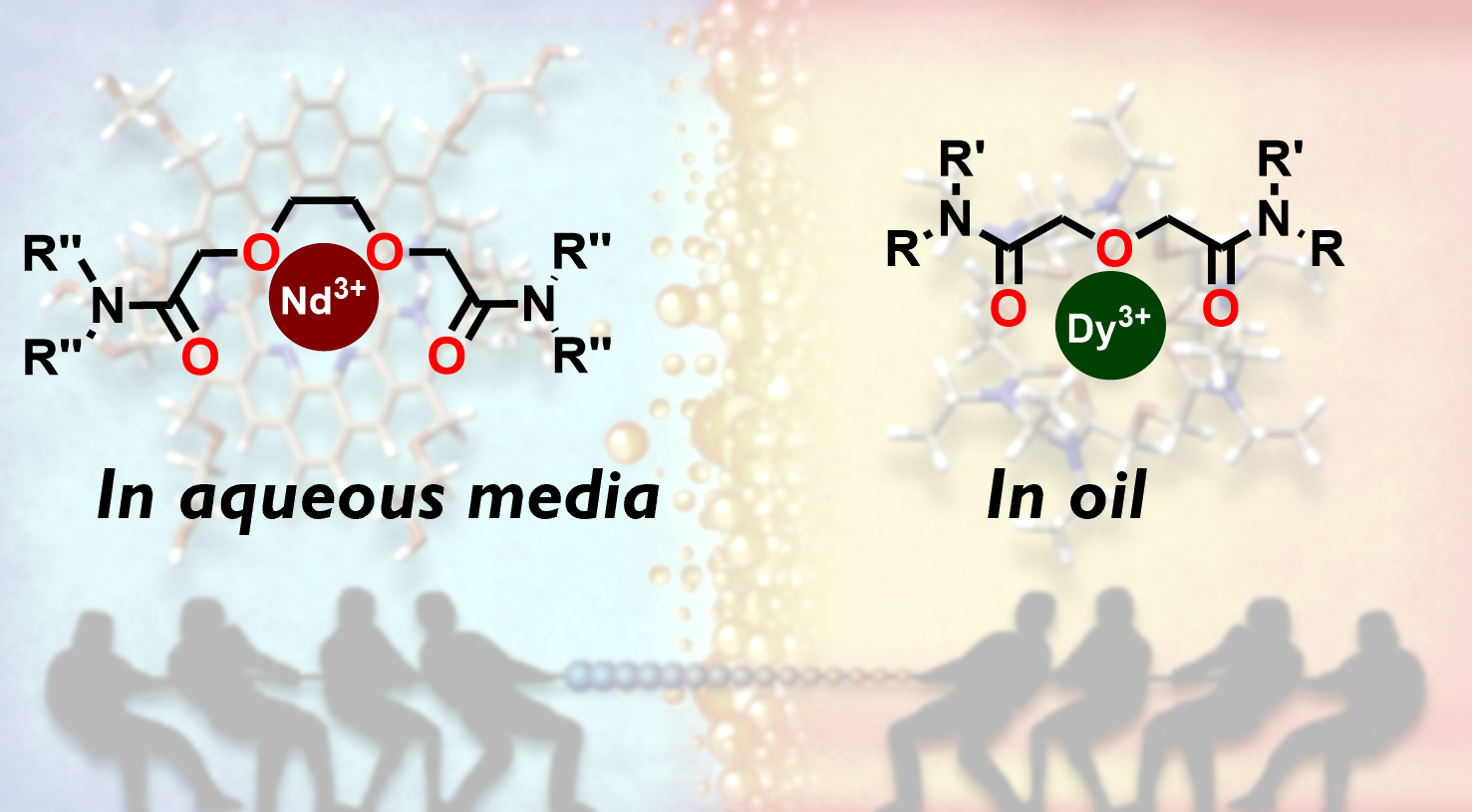
The use of water-soluble ligands in REE separation has been avoided due to their limited stability in acidic aqueous solutions. The new process uses aqueous alkali chloride media instead of hydrochloric acid, preserving ligand stability and allowing continuous recycling.
Achievement
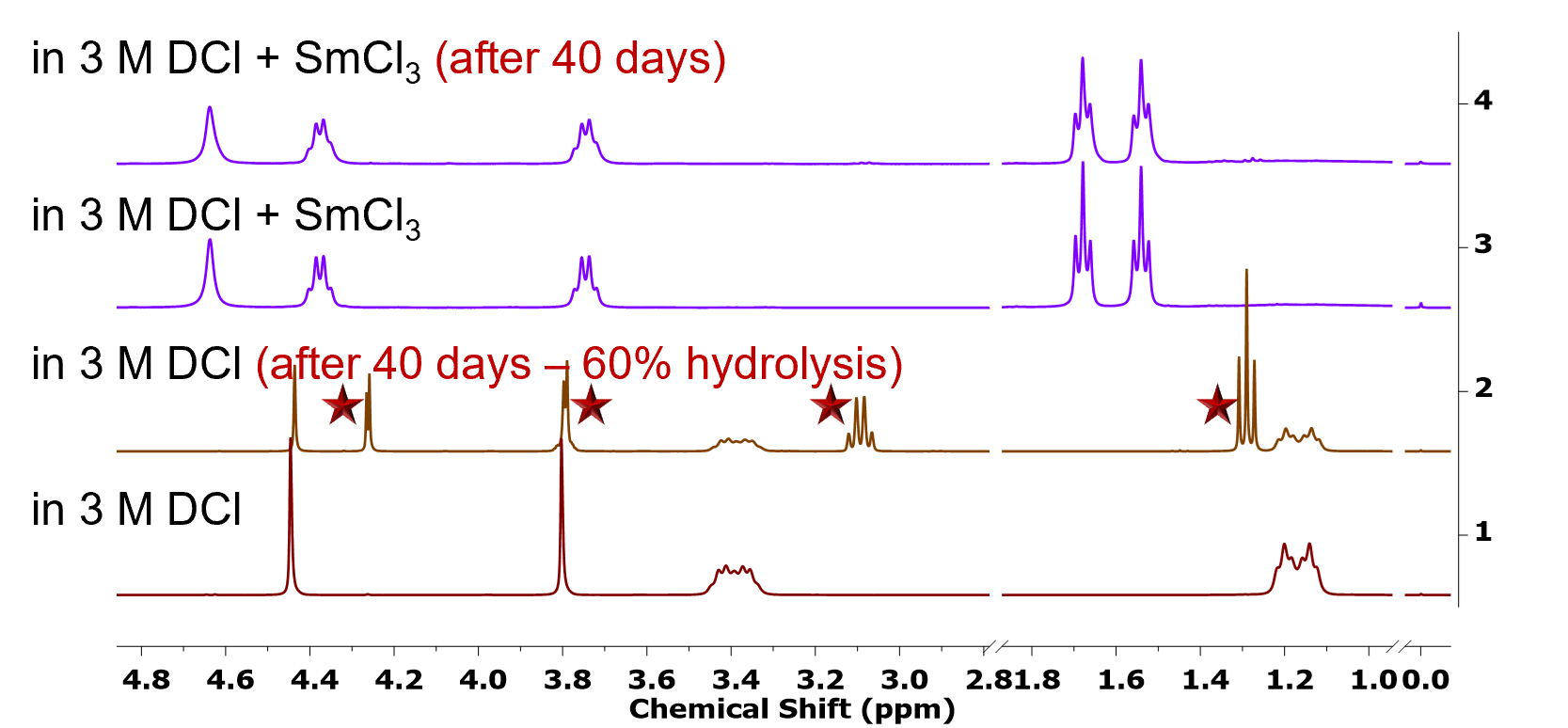
DOODA ligand maintains excellent chemical stability in 3-4 M alkali chloride media, with up to 0.3 M hydrochloric acid present. In contrast, 3 M HCl causes 60% ligand hydrolysis in just 40 days at 24 °C.
Significance and Impact
- At 0.1 M concentration in 2–4 M alkali chloride media, DOODA selectively recovers specific REEs from the oil-soluble DGA-6 phase.
- Two oil-soluble ligands then quantitatively re-extract these REEs, enabling continuous DOODA reuse.
Hub Target Addressed
Minimizing hazardous chemical use and waste generation.