This CMI-funded research was conducted at Lawrence Livermore National Laboratory and CMI industry partner GE
Achievement:
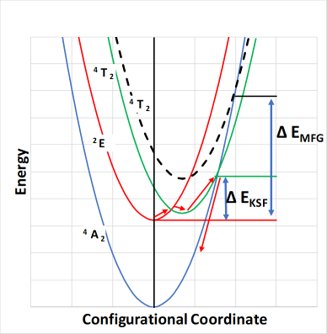
A comparative study of the decay probabilities and thermal quenching of Mn4+ luminescence in commercial MFG (oxide) and KSF (fluoride) phosphors is accomplished. Phosphor quenching temperature (Tq) is linked with the energy of the 4T2g state. The decay rate is faster in the oxide (MFG) phosphor.
Significance and Impact:
For application in LED, studying the resistance of Mn4+ QE to thermal impact is necessary because chip temperatures can approach 100 C-150 C.
A faster decay rate reduces photo-quenching with increasing flux (droop). By comparing the optical properties of two commercial Mn4+ phosphors, MFG and KSF, it was found that the thermal quenching is improved with increasing energy of the 4T2g state. The radiative decay rate of the oxide MFG is found to be much faster than the fluoride KSF phosphor.
Research Details and Next Steps:
- Tq (MFG;700 K) > Tq (KSF; 558 K)
- 4T2g is at 2.95 eV(MFG) & 2.75 eV (KSF)
- A single configuration coordinate model accounts for the higher Tq in MFG (see figure)
- Decay rate: MFG=165.8 s-1;KSF=54.6 s-1
- Measure droop in commercial MFG and KSF phosphors
