CMI researchers from Arizona State University conducted the activity for this highlight
Innovation
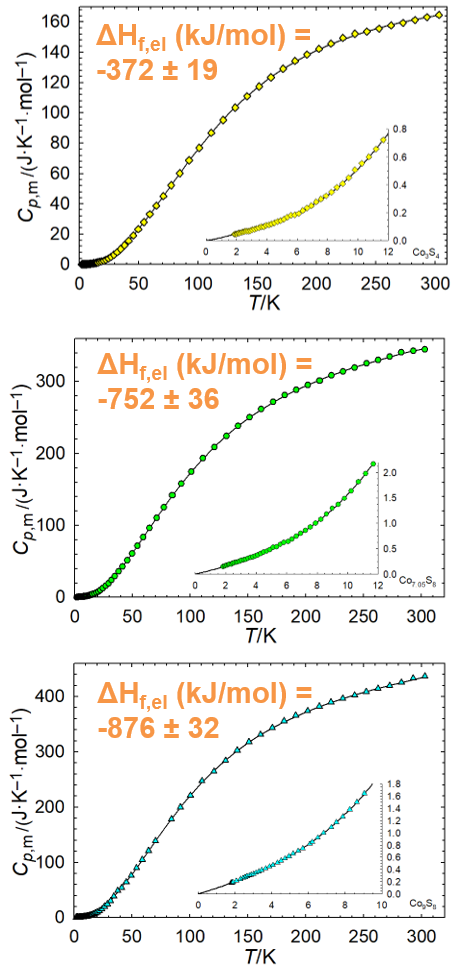
Synthesis and measurement of thermodynamic properties of sulfides Co9S8, Co7.05S8, and Co3S4. The goal is to present a set of recommended thermodynamic values for all solid cobalt sulfides stable at ambient conditions.
Achievement
Enthalpies of formation (from elements) of Co sulfides have been measured using high temperature oxide melt solution calorimetry. Low temperature heat capacity was measured by PPMS.
Significance and Impact
- Co demand is expected to outpace supply by the 2030s. New sources and better models are needed to optimize recovery. Sulfides are major co ore minerals, and understanding thermodynamic properties can help.
- Next steps: obtain high T heat capacity and melting temperatures, determine enthalpy of unquenchable Co4S3 from decomposition of other sulfides.
Hub Target Addressed
Developing and applying scientific tools to accelerate technology maturation.
