CMI at Arizona State University conducted the activity for this highlight
Innovation
Synthesize oxyhydroxides of Ni and Co with high temperature drop solution calorimetry to determine stability and decomposition conditions.
Achievement
Synthesize NiO(OH) and CoO(OH) and measure their enthalpies of formation and decomposition temperatures.
Significance and Impact
- CoO(OH) aka heterogenite is a common cobalt ore mineral. NiO(OH) is an important battery material.
- Next steps: synthesis of hexagonal CoO(OH) and y-NiO(OH), calorimetry, and heat capacity measurements.
Hub Target Addressed
Developing and applying scientific tools to accelerate technology maturation.
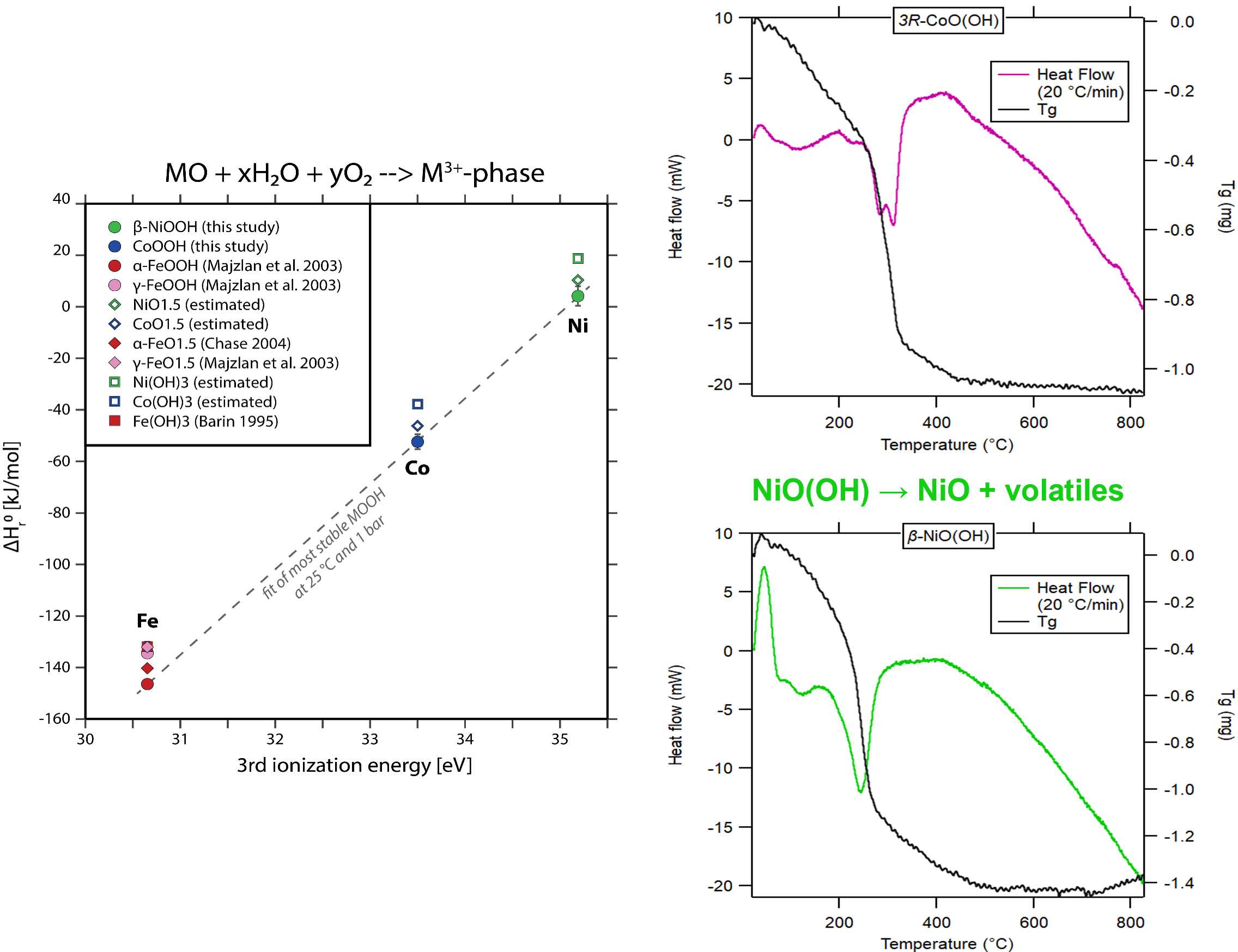
Top & Bottom Right: Scanning calorimetry and thermogravimetry showing decomposition conditions for Co and Ni oxyhydroxides as a function of temperature.
