CMI researchers from Ames National Laboratory, Lawrence Livermore National Laboratory and Loukus Tech conducted the activity for this highlight
Innovation
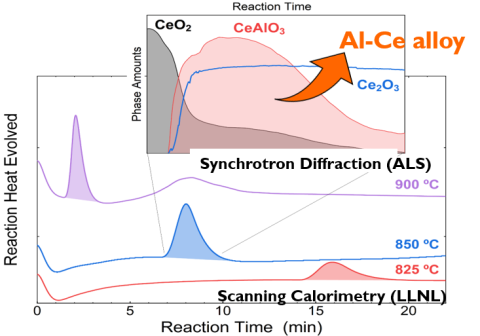
Understanding of the chemical reaction mechanism for the reduction of CeO2 by aluminum enabled a 95% yield of an Al-Ce alloy supporting an energy efficient process for domestic production.
Achievement
First in-situ study of rapid high-temperature direct reduction process reveals multi-step reaction mechanism.
Significance and Impact
Understanding reaction steps enables design of industrial process for high yield at reduced energy consumption. This could reduce alloy cost by 20-50%, improving economics for converting low-value CeO2 into high-strength Al-Ce, while supporting domestic rare earth mining and advanced alloy production.
Hub Target Addressed
Recovering and converting critical materials into high value refined products.
Published in: Materials Horizons 2024 doi.org/10.1039/D4MH00087K