CMI at Arizona State University conducted the activity for this highlight
Innovation
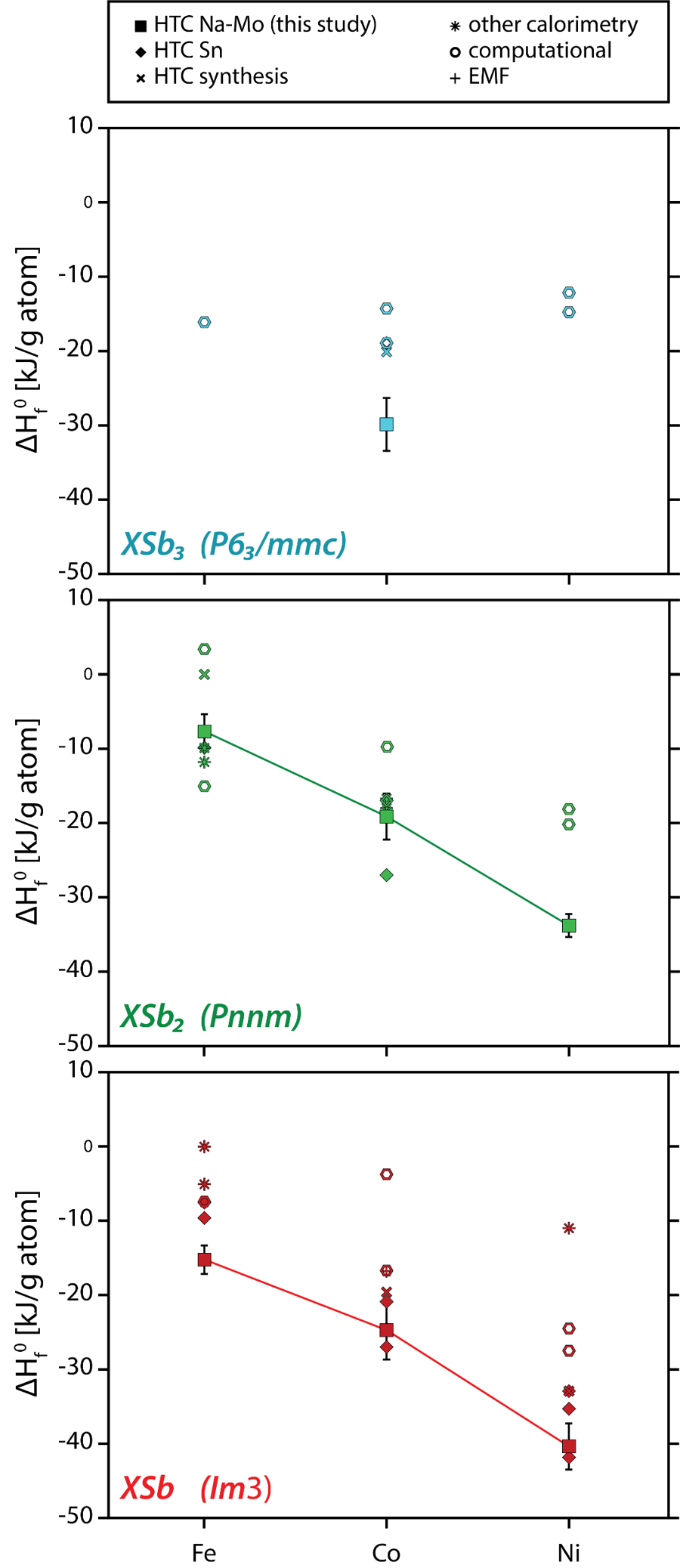
Synthesis and measurement of thermodynamic properties of Ni, Co and Fe antimonides by oxidative drop solution calorimetry.
Achievement
Long-term solid-state synthesis (3-5 months) ensures full homogeneity of
NiSb, CoSb, FeSb, NiSb2, CoSb2, FeSb2 and CoSb3. Enthalpies have been fully constrained for all samples.
Significance and Impact
- Antimonides are crucial for energy transition industry but also occur naturally in important ore deposits. Their stability would aid in improving our understanding of critical mineral ore deposit formation. However, it is not well understood which arises from the lack of well constrained thermodynamics.
- Next steps: obtain high T heat capacity by DSC, obtain low T heat capacity and entropy by PPMS, determine thermal stability under air and inert atmosphere.
Hub Target Addressed
Developing and applying scientific tools to accelerate technology maturation.