The overarching goal of this collaborative research is to (a) elucidate the fundamental interfacial interactions between active sites, supports, reactants, and reaction media, and (b) use this understanding to create materials with the best properties of homogeneous and heterogeneous catalysts for efficient reductive transformations. The main hypothesis is that these interactions can be harnessed to mediate more challenging transformations under less forcing reaction conditions. Their effects, both individual and collective, on catalytic properties are systematically studied by combining selected sites and 3D-ordered mesoporous supports. Inert supports provide isolated sites, whose performance is being compared to those constructed with non-innocent surfaces that participate through substrate and reagent activation. This work combines complementary expertise in synthetic techniques, rigorous spectroscopic characterization, and mechanistic studies. Kinetic experiments and surface-sensitive dynamic nuclear polarization (DNP) solid-state NMR methods are used to benchmark interfacial catalysts with those lacking cooperativity, such as homogeneous sites or catalytic sites on inert supports. The project focuses on reduction of carbonyls (ketones, esters, acids, and amides) and phenols through hydrogenation or hydroboration, or in tandem multicatalytic reactions. These functional groups are abundant in biorenewable compounds and energy-relevant molecules, whose conversion suffers from a lack of selective and energy efficient catalytic processes.
This research was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, Chemical Sciences, Geosciences, and Biosciences Division, Catalysis Science program. Ames National Laboratory is operated for the U.S. Department of Energy by Iowa State University under Contract No. DEAC02-07CH11358.
Principal Investigator: Aaron Sadow
Co-PIs: Frederic Perras, Takeshi Kobayashi, Long Qi, Damien Culver
Staff Scientist: Brennan Walder, Yuting Li
Post Doc: Zhenqi Zhao
News & Highlights
 Research Highlight
Research Highlight
Sensitive NMR Method Reveals Motions of Surface Sites
 Research Highlight
Research Highlight
17O NMR Facilitates Structural Studies of Catalytic Sites
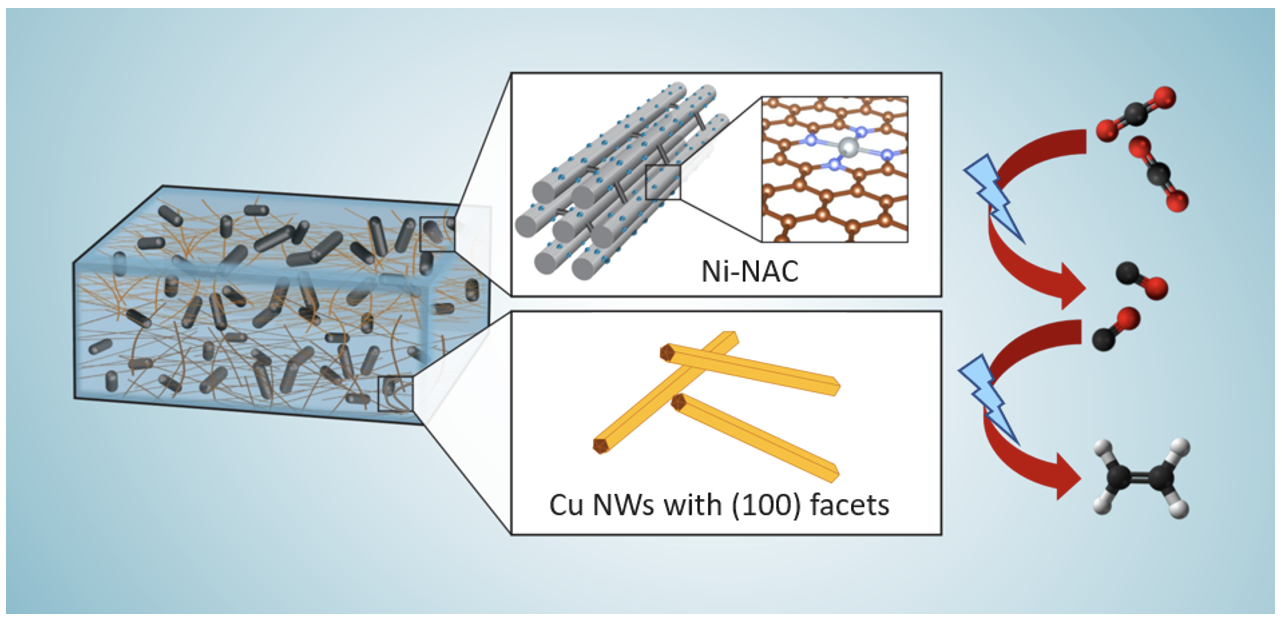 Research Highlight
Research Highlight
Earth-Abundant Metals Catalyze Energy-Efficient Carbon Dioxide Removal (CDR)
 Research Highlight
Research Highlight
General Synthetic Strategy to Ordered Mesoporous Carbon Catalysts with Single-Atom Metal Sites for Electrochemical CO2 Reduction (CO2RR)
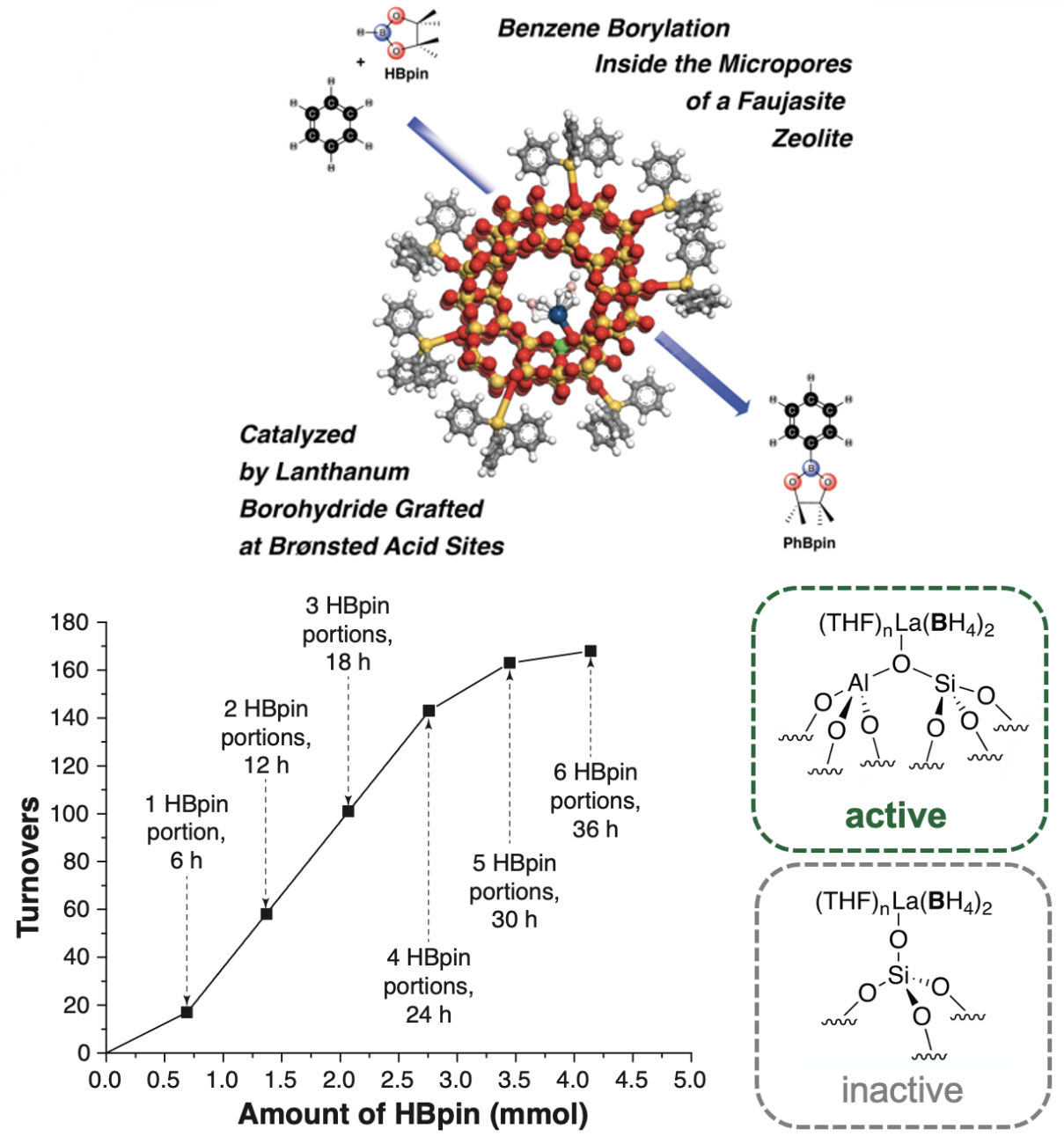 Research Highlight
Research Highlight
