In the quest for domestic sources of lithium to meet growing demand for battery production, scientists at the Department of Energy’s Oak Ridge National Laboratory are advancing a sorbent that can be used to more efficiently recover the material from brine wastes at geothermal power plants.
Domestic production of lithium, the lightest of elemental metals, is considered a priority for the nation. It is essential for the manufacturing of lithium-ion batteries commonly used for everything from electric vehicles to cell phones and laptops. Yet it is sourced almost exclusively from other countries, either concentrated using a solar evaporation process from natural brine sources or recovered from ore. The United States imported 4,000 metric tons of lithium in 2018, according to the U.S. Geological Survey, a figure expected to grow exponentially.
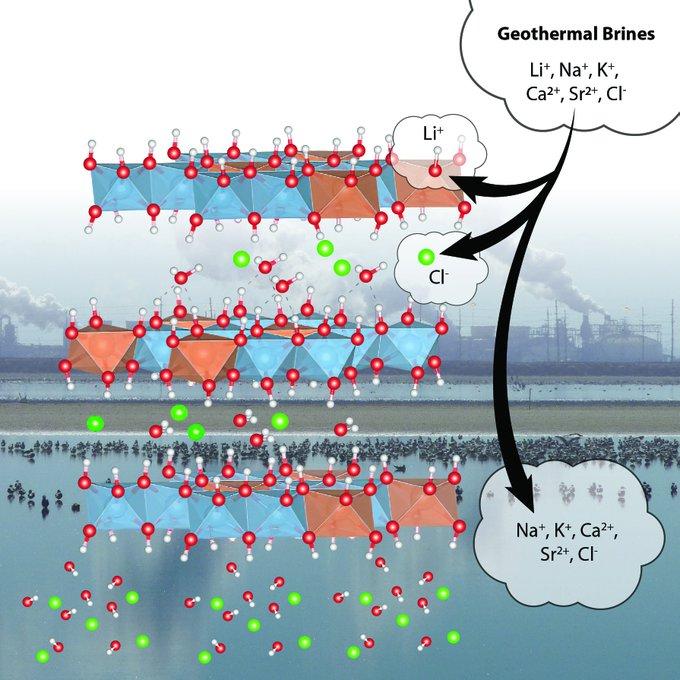
In work for DOE’s Critical Materials Institute, scientists at ORNL are working to refine a sorbent that can more effectively recover lithium salts from concentrated brines at geothermal plants. These plants pump hot water from geothermal deposits and use it to generate electricity. Concentrated brines left over from the operation are then pumped back into the ground.
ORNL and its research partners are working to improve the capacity and selectivity of a sorbent that could extract the lithium from these brines. The lithium-aluminum-layered double hydroxide chloride (LDH) sorbent they’re developing is a low-cost, reusable option for large-scale industrial plants.
The sorbent’s thermochemical properties were also characterized using differential scanning calorimetry and thermogravimetry at the CMI Team member University of California-Davis. The tests explored how the ordering of water molecules in the sorbent has a direct impact on the material’s stability and effectiveness. The scientists confirmed that by replacing some of the aluminum with iron in the sorbent, the material is made more thermodynamically stable and can be used as an alternate sorbent for extracting lithium. The work was described in a paper in The Journal of Physical Chemistry C.
By putting the sorbent through these paces, “we get valuable information on how water is accommodated in the sorbent’s layers and how that dictates separation and stability,” said Parans Paranthaman, principal investigator for the project and leader of the Materials Chemistry Group at ORNL.
The work is being conducted with CMI Team industry partner All American Lithium, which is seeking to refine the technology in preparation for a commercial lithium plant in California.
For complete information, link to the story on the ORNL website: https://www.ornl.gov/news/ornl-develops-sorbent-recover-lithium-geothermal-brines
