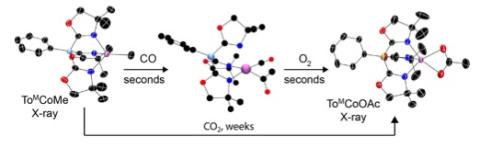
Acetate, a biological energy carrier, is formed from the combination of carbon monoxide and a methyl group followed by oxygenation. This kind of sequence was observed in a new synthetic cobalt complex, and it is also well known as part of the Krebs cycle in natural systems. Acetate might also be formed from the direct addition of a methyl group and naturally abundant carbon dioxide, yet nature chooses an energetically less efficient route. Why? Our studies of the synthetic cobalt system indicate that both routes yield acetate, but the pathway involving carbon monoxide is kinetically preferred over carbon dioxide by more than 106 times (over six orders of magnitude, essentially a difference between instantaneous reaction and 2 weeks!). Living organisms cannot wait for the slow reaction, and instead enzymes have developed a fast, low temperature, mild alternative. This idea also applies in synthetic catalytic chemistry, in which carboxylate moieties are installed by the combination of carbon monoxide with an organometallic compound followed by oxidation, rather than direct treatment with carbon dioxide. That is, approaches for utilization of carbon dioxide as a C1 reagent in catalytic synthesis might be advantaged by a three-step process involving reduction to carbon monoxide, reaction with organometallics, followed by oxidation.
We envision and are working toward new catalytic processes for hydrocarbon conversion to carboxylic acids developing from this work. This proposed new catalytic transformation would involve our process for formation of acetate from methyl, carbon monoxide, and oxygen coupled to the synthesis of metal methyl compounds from hydrocarbons and metal acetate. This kind of hydrocarbon functionalization scheme, when realized, would provide new and efficient production of acetic acid and other carboxylic acids directly from abundant, energy-rich hydrocarbons.
Reinig, R. R.; Fought, E. L.; Ellern, A.; Windus, T. L.; Sadow, A. D. “Rapid and ordered carbonyl and oxygenation of a cobalt(II) methyl” Chem. Commun. 2017, 53, 11020.
