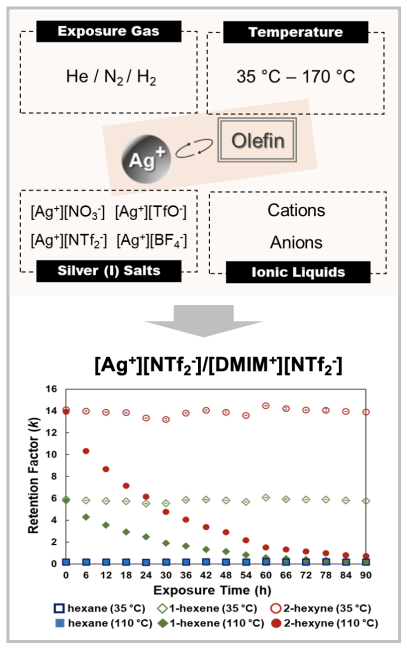
Long-term olefin selectivity offered by silver salt/ionic liquid (IL) mixtures is evaluated by inverse gas chromatography (IGC). The approach enables the separation media to be examined under various environmental conditions (e.g., different temperatures and gas streams) typically encountered when performing olefin separations.
The versatile nature of IGC permits the temporal stability of silver(I) ions to be studied. The results can be used to guide the development of optimal silver salt/IL mixtures that offer high olefin selectivity in a variety of separation systems.
- Length of alkyl chain substituents in IL cations do not influence the long term stability of silver(I) ion.
- Stronger silver-olefin coordination is observed when silver salts are dissolved in ILs featuring long alkyl chain substituents.
- Silver(I) ion from the [Ag+][NTf2-] salt coordinates most strongly to olefins when dissolved in ILs containing the [BF4-] anion.
- Silver(I) ions are more stable in [NTf2-]-based ILs than [BF4-]-containing ILs.
- The [Ag+][NTf2-]/[DMIM+][NTf2-] mixture provided stable olefin/paraffin separation performance when exposed to hydrogen at low temperatures (35 °C). Olefin selectivity diminished gradually upon hydrogen exposure at temperatures exceeding 100 °C.
Eor, P.; Ryoo, D.; Nan, H.; Anderson, J.L. "Characterizing Olefin Selectivity and Stability of Silver Salts in IonicLiquids Using Inverse Gas Chromatography." ACS Omega 2020, 5, 31362–31369
