The fluctuations, associated with quantum-critical points, i.e., second-order phase transitions at zero temperature, have been considered crucial for the stabilization of intriguing phenomena, such as superconductivity or non-Fermi liquid behavior. This motivates the search for novel states by tuning magnetic phase transitions towards 0 K by external parameters, such as physical pressure or chemical substitutions. For clean metallic ferromagnets, it is widely believed that quantum criticality is avoided for generic reasons. It was proposed that the metallic ferromagnet LaCrGe3 is a prime example to study the consequences of the avoidance in laboratory experiments. In this study, the authors revisited the temperature-pressure phase diagram of LaCrGe3 by combining thermodynamic (specific heat and thermal expansion), transport, x-ray and neutron scattering as well as muon-spin resonance measurements. In addition to discovery of a new phase line, the results strongly suggest that a short-range magnetically ordered cluster phase emerges as the ferromagnetic transition is suppressed towards zero temperature. This insight points towards the influence of disorder that is inevitably present even in stochiometric compounds.
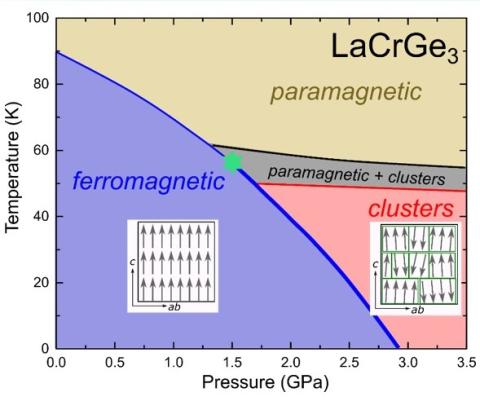
“Formation of short-range magnetic order and avoided ferromagnetic quantum criticality in pressurized LaCrGe3” Elena Gati, John M. Wilde, Rustem Khasanov, Li Xiang, Sachith Dissanayake, Ritu Gupta, Masaaki Matsuda, Feng Ye, Bianca Haberl, Udhara Kaluarachchi, Robert J. McQueeney, Andreas Kreyssig, Sergey L. Bud'ko, and Paul C. Canfield, Phys. Rev. B 103, 075111 (2021): https://doi.org/10.1103/PhysRevB.103.075111 (Editor’s suggestion)
