A newly published review in the Royal Society of Chemistry’s journal Nanoscale marks the milestones in research and development of metal nanoparticles encapsulated or intercalated under the surface of a layered 2D material, along with opportunities for future growth in the field.
It has been three short years since a team of Ames Laboratory scientists discovered a strategy to
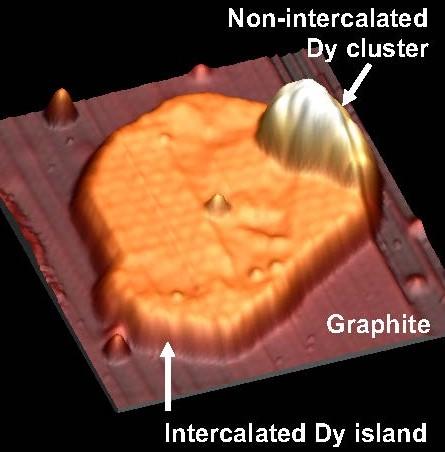
sheathe dysprosium nanoparticles beneath the surface of graphite. The strategy utilized ion bombardment to create defects on the graphite surface, and deposition of metal from the vapor phase onto the heated graphite surface, in an ultra-high vacuum environment. Because of the interplay between the unique electron behaviors of both layered 2D materials and metal nanoparticles, in combination they hold great promise in applications such as quantum computing, solar cells, catalysis, and sensing.
“We’ve come a long way from that first discovery,” said Ann Lii-Rosales, a postdoctoral research associate at CU Boulder, who performed much of the experimental work at Ames Laboratory as a graduate student. “We’ve been able to accomplish this encapsulation with a variety of metals, including copper, iron, ruthenium, platinum, and gadolinium. The encapsulation turned out to be a general phenomenon. We’ve been able to observe how the metals grow under the graphene skin of graphite. But we need to further understand how that process happens, and how we can control it in order to achieve useful properties.”
In addition to experimental contributions, expanding theoretical understanding of the process is also important. James Evans, senior scientist at Ames Laboratory’s Division of Chemical and Biological Sciences, said that future theory and simulation research opportunities were many.
“Significant progress has been made characterizing the energetics of intercalation at Ames Laboratory through DFT studies by Yong Han, and the group of C.-Z. Wang. However, our studies of the kinetics of the overall intercalation process, while already providing useful insights, are still in their infancy,” Evans said.
“There’s a rule book for the properties of bulk, or three-dimensional materials—and it contains big chunks that are universally understood and accepted. But the rule book for 2D materials is largely unwritten. There are lots of things we don’t know. We get lots of surprises, and then we must explain them,” said principal investigator and Ames Laboratory scientist Pat Thiel in 2016 of her work. Thiel, a fellow of the American Academy of Arts and Sciences, physical chemist, materials scientist, and Distinguished Professor at Iowa State University, passed away in September 2020 after a long battle with breast cancer.
The review stands in part as a testimony to Thiel’s dedication and progress in writing that rule book, collaborating with many other researchers along the way.
Thiel was internationally renowned for her studies of the formation and stability of metal nanostructures on surfaces, as well as for ground-breaking analysis of the surface structure of quasicrystals. This work on metal intercalation in the last few years extended these efforts and opened a novel and rich area of research.

The review, “Encapsulation of Metal Nanoparticles at the Surface of a Prototypical Layered Material,” is authored by Ann Lii-Rosales, Yong Han, Dapeng Jing, Michael C. Tringides, Scott E. Julien, Kai-Tak Wan, Cai-Zhuang Wang, King Chun Lai, James W. Evans and Patricia A. Thiel; and published in Nanoscale.
Work was performed using a grant of computer time at the National Energy Research Scientific Computing Centre (NERSC). NERSC is a DOE Office of Science User Facility.
Ames Laboratory is a U.S. Department of Energy Office of Science National Laboratory operated by Iowa State University. Ames Laboratory creates innovative materials, technologies and energy solutions. We use our expertise, unique capabilities and interdisciplinary collaborations to solve global problems.
Ames Laboratory is supported by the Office of Science of the U.S. Department of Energy. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit https://energy.gov/science.
Contacts:
Ann Lii-Rosales, Dept. of Chemistry, University of Colorado Boulder, 614-940-3572
James Evans, Division of Chemical and Biological Sciences, 515-294-1638
Laura Millsaps, Ames Laboratory Communications, 515-294-3474
