Lithium-ion batteries are everywhere, in cell phones, computers, electric vehicles, and even toys, to name only a few places. They have become an integral part of our everyday lives.
Lithium, the element that makes these batteries possible, is considered a critical material by the U. S. Department of Energy, because it is essential in many energy applications, creating high demand and the risk of disrupted supplies.
Despite their wide use, it is estimated that only 5% of lithium batteries are currently recycled. Because lithium has high supply risk, discarded batteries are a potential source for recovering lithium. Scientists are developing improved ways to recycle and recover some of that lithium. Typical methods for recycling these batteries require harsh liquid chemicals or heat to complete the process. These processes can produce toxic byproducts and require large amounts of energy.
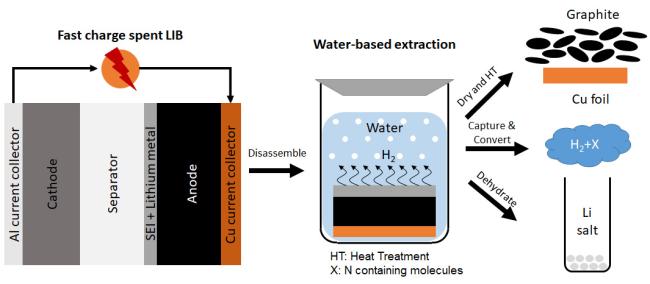
Recently, a team of scientists from the U. S. Department of Energy Ames National Laboratory developed a new recycling process that eliminates the need for chemicals and high heat. This process, the Battery Recycling and Water Splitting (BRAWS) technology, uses only water and carbon dioxide to complete the process. It does not require chemicals or heat and allows scientists to recover more lithium from spent batteries than other recycling methods.
According to Ikenna Nlebedim, a scientist at Ames Lab and leader of the research team, the three typical methods for lithium-ion battery recycling are hydrometallurgical, pyrometallurgical, and direct recycling. Hydrometallurgical methods grind spent batteries and dissolve the resulting materials in acid before extracting recyclable elements using different chemicals. Pyrometallurgical methods melt waste at high temperatures to extract recyclable materials. Direct recycling involves physically taking apart and collecting materials that can be reused from spent batteries, grinding, and recovering materials from the ground waste.
“Each of these processes has their advantages and disadvantages,” said Nlebedim. “So, we try to create something that integrates the advantages and leaves out the disadvantages.”
When lithium-ion batteries undergo fast charging, they do not last as long because the fast charging causes the lithium to build up on the anode (positive side of the battery electrode). Over time, the lithium build-up causes the battery to fail.
The first step in the BRAWS technology is to use a set of protocols that includes fast charging to force as much additional lithium as possible to build up on the battery anode. Next, the battery is dismantled. “Right now in the lab, we're manually dismantling the battery,” said Nlebedim.

After dismantling the battery, the anode, typically made of graphite, is then immersed in water, and CO2 is added to recover lithium as lithium carbonate. This step results in recovery of almost all the lithium from the original battery and produces green hydrogen as a byproduct.
“Because lithium is very reactive, when we put that anode in water, it divides the water molecule by stripping the oxygen and producing hydrogen as a gas, which can be recovered safely and used as a fuel,” said Nlebedim. “Carbon dioxide is consumed in the process, so it has the added benefit of cleaning up the environment.” This technology does not rely on chemicals or extreme temperatures, so other materials can be extracted in a form that can be directly reused.
“Recycling tends to be something that requires economic sustainability. One way to sustain recycling is to make profit with everything you can. Recovering lithium, other parts of the battery, and producing green hydrogen at the same time, strengthens the economics of our process” said Nlebedim.
Ames National Laboratory is a U.S. Department of Energy Office of Science National Laboratory operated by Iowa State University. Ames Laboratory creates innovative materials, technologies, and energy solutions. We use our expertise, unique capabilities, and interdisciplinary collaborations to solve global problems.
Ames Laboratory is supported by the Office of Science of the U.S. Department of Energy. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, please visit https://energy.gov/science.
