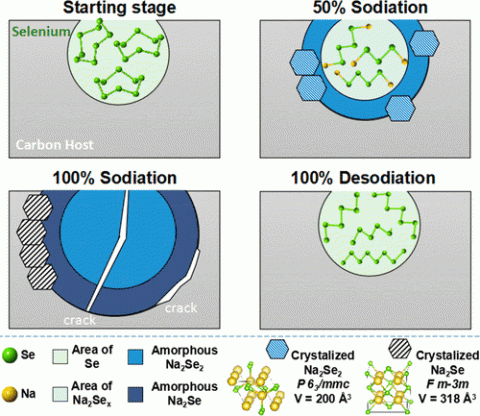
Sodium metal batteries are a promising new technology for grid storage applications with the potential to store over twice the energy as conventional lithium ion batteries. Selenium’s superior electrochemical properties, when compared with sulfur, favor its inclusion into sodium ion battery cathodes. The sodiation mechanism, replacement of selenium for sodium, in Se-based sodium ion batteries was, however, poorly understood. A combined advanced electron microscopy, electroanalytical techniques, and solid-state nuclear magnetic resonance spectroscopy study revealed the inner workings of selenium-impregnated microporous carbon host cathode materials during cycling. Counter to expectations, it was discovered that the sodiation mechanism is both inhomogeneous in speciation and location. More specifically, it was found that selenium does not undergo a sequence of discrete crystalline phase transitions but rather is statistically converted into arrays of polyselenide compounds of varying sizes. These polyselenides are inhomogeneously distributed within the pores of the host, with the shorter, more reduced, anions located in proximity with the carbon interface, suggesting that they are the first to be reduced. The overall sodiation–desodiation sequence in pore-confined Se structures appears more complex than the transformations described in the literature for unconfined nanoscale Se.
F. A. Perras, S. Hwang, Y. Wang, P. Liu, R. Biswas, S. Nagarajan, V. H. Pham, J. A. Boscoboinik, D. Su, M. Pruski, D. Mitlin , Site-Specific Sodiation Mechanisms of Selenium in Microporous Carbon Host, Nano Lett., 20, 918-928, (2020). DOI: 10.1021/acs.nanolett.9b03797
