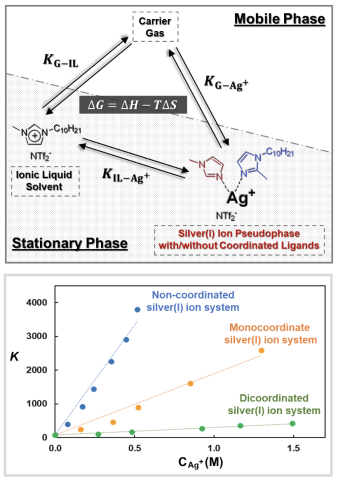
A three-phase chromatographic model treating silver(I) ions as a pseudophase is constructed and applied to investigate the olefin separation mechanism in silver(I) salt/ionic liquid (IL) mixtures. The partition coefficients and the values for enthalpy, entropy, and free energy of solvation of analytes are determined using the model.
Significance and Impact
The developed model allows for temporal chemical changes of silver(I) ions in separation systems to be measured and optimization of silver(I) salt/IL mixture compositions for high olefin selectivity.
Research Details
- Alkenes, alkynes, and dienes interact strongly with the silver(I) salt/IL mixtures primarily due to their partitioning towards the silver(I) ion pseudophase from the gas phase (high 𝐾_(〖G−Ag〗^+ ) values).
- Aldehydes, ketones, esters, and aromatics partition strongly to the IL solvent and silver(I) ion pseudophase.
- As more imidazole-based ligands are coordinated to silver(I) ions, the interaction between the silver(I) ion pseudophase and analytes weakens, resulting in lower 𝐾_(〖G−Ag〗^+ ) and 𝐾_(〖IL−Ag〗^+ ) values.
- The interaction of olefins within the silver(I) salt/IL mixtures is enthalpically dominated, while ligand coordination to the silver(I) ion results in variations for both enthalpic and entropic contributions to the free energy of solvation.
Eor, P.; Anderson, J.L. "Using a Chromatographic Pseudophase Model To Elucidate theMechanism of Olefin Separation by Silver(I) Ions in Ionic Liquids," Analytical Chemistry, 2021, 93, 13284-13292.
