The traditional picture of metal surfaces involved in chemisorption and catalysis processes is that they are static or frozen. However, in-situ observations under certain reaction conditions reveals their dynamic nature. Also, it has long been recognized that metal nanocluster dynamics can be deleterious leading to coarsening and thus catalyst degradation. Other studies have shown an extraordinary acceleration in the destabilization of metal nanostructures at surfaces in the presence of even trace amounts of additives. In the latter cases, the presence of metal-additive complexes have been implicated in enhanced surface dynamics.
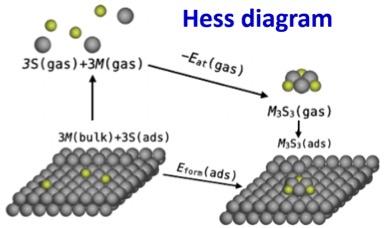
Thus, systematic investigation of the stability of such complexes on surfaces is needed. We develop a Hess diagram framework to systematically compare the energetics of complex formation on surfaces with that in the gas-phase. In this way, we can dissect the various contributions to stability, and identify the role of interaction with the surface and the source of the constituent atoms in modifying gas-phase stability.
Interpretation of experiment can be subtle due to dependence of the relevant formulation of formation energy on the experimental conditions. Our analysis accounts for such subtleties to provide the appropriate interpretation of a variety of claimed experimental STM observations of complexes.
Da-Jiang Liu, Jiyoung Lee, Theresa L. Windus, Patricia A. Thiel and James W. Evans, Stability of M3S3 complexes on fcc M(111) surface: M = Au, Ag, Cu, and Ni. Surface Science 646 (2018) 2-8.
