 Scientific Achievement
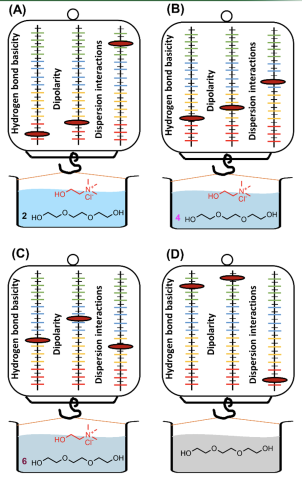
Scientific AchievementSolvation characteristics of deep eutectic solvents (DESs) comprised of choline salts as the hydrogen bond acceptor (HBA) and polyols as hydrogen bond donor (HBD) were thoroughly characterized using gas chromatography. Solvation models were constructed for DES systems popularly employed in catalysis, chemical separations, and organic synthesis to describe variations in their individual solvation interactions when their HBA/HBD ratio is systematically modulated.
This study emphasizes that each molar composition of DES offers a unique solvation character that is largely different from that of its starting materials, which can significantly influence its efficacy as a catalyst or reaction medium. The models developed in this study enable us to predict the performance of DESs in catalysis and separation systems based on their solvation characteristics. This advancement provides a framework for the design and preparation of task-specific DESs for separations on the basis of their solvation properties in order to minimize energy consumed for large industrial scale applications.
- Choline chloride [Ch+][Cl-] and choline acetate [Ch+][Ace-] can form DESs with polyols comprised of several different HBA/HBD ratios ranging from 1:2 to 1:10 and each composition possesses a unique set of solvation properties.
- Hydrogen bonding interactions varied more drastically with small changes in HBA/HBD ratio compared to dispersive-type and dipolar interactions.
- Higher molar ratios of HBD in DESs result in stronger hydrogen bond acidity while starting materials comprised of neat HBDs interact the most with basic analytes.
- DESs comprised of 1:4 molar ratio of HBD exhibited the strongest interactions with acidic analytes such as alcohols while triethylene glycol and 1,8-octane diol employed as starting materials possessed the lowest hydrogen bond basicity.
Abbasi, N.M., Farooq, M.Q., Anderson, J.L. “Investigating the effect of systematically modifying the molar ratio of hydrogen bond donor and acceptor on solvation characteristics of deep eutectic solvents formed using choline chloride salt and polyalcohols.” J. Chromatogr. A 2022, 1667, 462871.
