Degradation of supported metal catalyst nanoclusters (NCs) often occurs via Ostwald Ripening (smaller NCs decay transferring their mass to larger NCs). This process can be enhanced by the environment under operating conditions. It has been suggested that the formation of volatile metal-additive complexes underlies this effect. These are potentially more effective at mass transport than metal atoms. However, in
complex catalytic systems, it is difficult to provide a definitive analysis of the mechanism underlying enhanced coarsening.
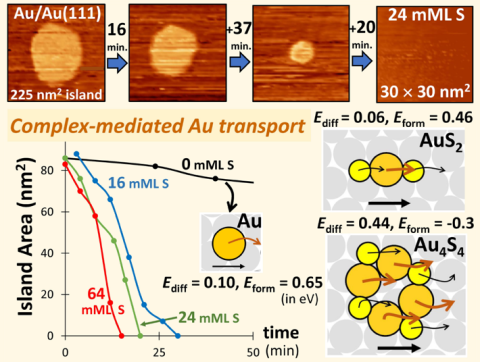
In contrast, in well-controlled ultra-high vacuum model systems with perfect single-crystal surfaces and controlled exposure to additives, such analysis is viable. We choose Au(111) surfaces given the central role which these play in surface functionalization, formation of self-assembled monolayers, and in catalysis (as well as the prominence of {111} facets on Au nanoparticles). In addition to detailed experimental characterization, these systems enable high-level theoretical analysis. The effective barrier, Eact = Eform + Ediff for NC decay in Ostwald Ripening is determined not just by the density of mass carriers (which is controlled by their formation energies, Eform), but also the barrier for surface diffusion of these mass carriers, Ediff. Both these energies can be reliably determined from Density Functional Theory analysis for not just Au atoms (the default mass carrier), but also for a variety of Au-S complexes to determine which has the lowest effective barrier, and thus would likely dominate mass transport. This information provides key input to modeling of the kinetics of enhanced Ostwald ripening.
Scientific Achievement:
Significance and Impact:
Research Details:
Peter M. Spurgeon, Da-Jiang Liu, Theresa L. Windus, James W. Evans, Patricia A. Thiel, Enhanced nanostructure dynamics on Au(111) with adsorbed sulfur due to Au-S complex formation, ChemPhysChem, 2021, 22, 349-358. Journal Front Cover.
