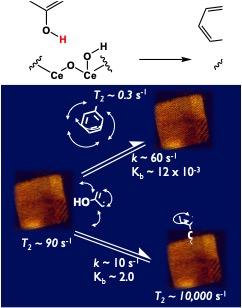
A key challenge for understanding the mechanism of solid-liquid interfacial catalysis is to be able to measure the kinetics and equilibria of each elementary steps involved in a transformation. Among those elementary steps, the rate and equilibrium of reactant binding to the surface of a catalyst is particularly difficult to assess in presence of competing solvent molecules. To address this challenge one can monitor the mobility of reactant molecules in a suspension of catalysts. By closely following changes in nuclear magnetic relaxation rates one can deduce the fraction of molecules bound to catalyst surfaces and the rate at which binding events happen. An additional complication though, is that measurement of nuclear magnetic relaxation requires long acquisition times, over which heterogeneous catalysts tend to settle down in sample tubes. To overcome this problem, in collaboration with ISU faculty, we developed a method for suspending heterogeneous catalysts in sample tubes by trapping them inside agarose gels. The pore sizes of the gel are large enough to entrap yet allow catalyst particles to tumble without sedimenting and thereby enable sufficient data collection to fit to models of proton relaxation.
This method allowed identification of two different and sequential modes of phenol binding to the surface of ceria nanocubes, each one with distinct rate and equilibrium constants. Importantly, these new results support and provide quantitative data on our previously published findings on the role of solvent on reactant binding in solution. Future work will involve correlating catalytic activity and selectivity to the kinetics and geometry of binding. Understanding the behavior of reactions in water is critical to enable novel transformations of biorenewables under conditions that maximize their energy efficiency.
