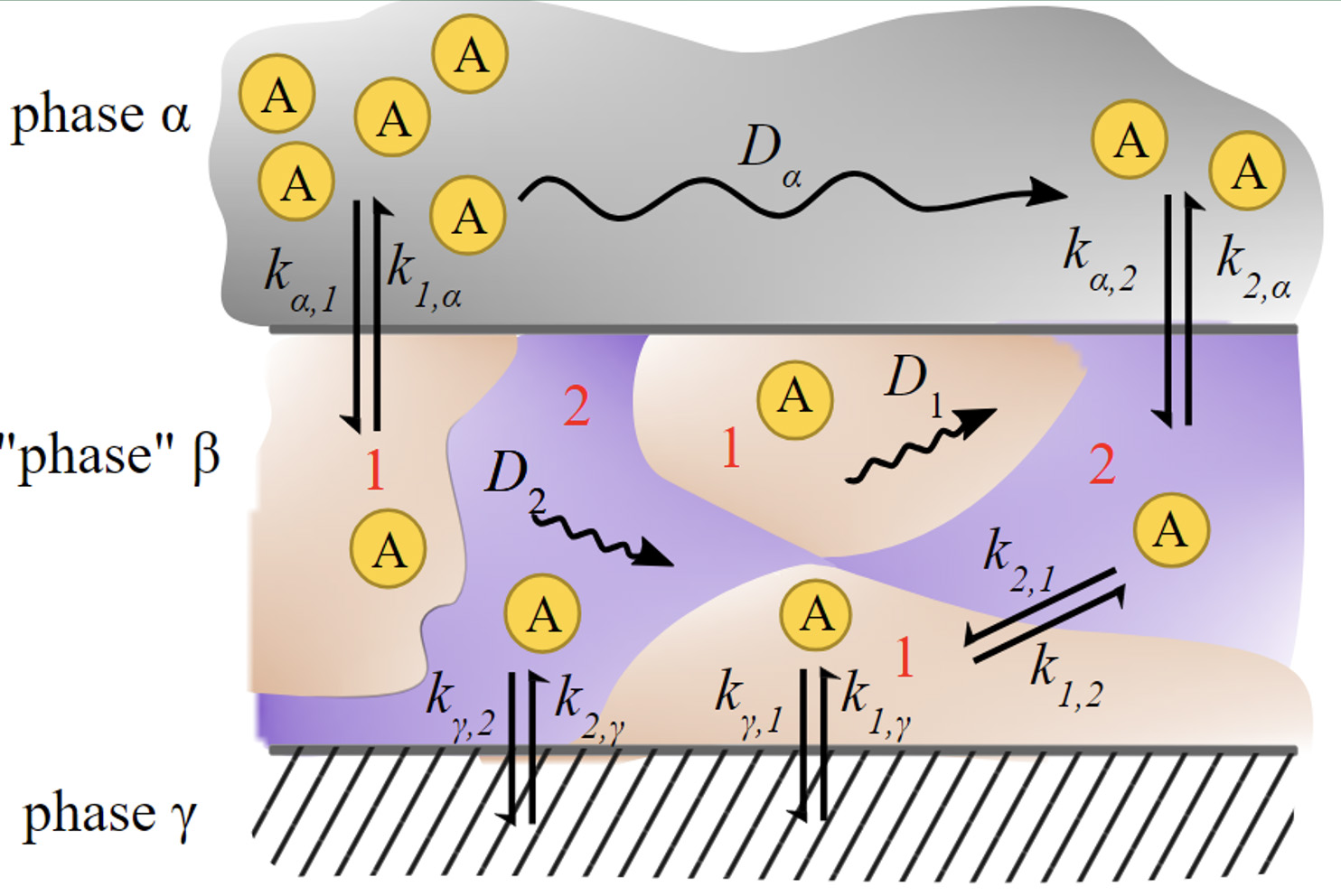
Previous investigations have focused on the small (~1-nm) domains formed by ionic liquids. We observe two large domains in tetradecyltrihexyl phosphonium chloride that are stable up to 50˚C, which is attributed to the critical point for a liquid-liquid phase transition.
Ionic liquids (a class of liquid salts) have exceptional properties in separations that may benefit the DOE mission in sequestering CO2 or rare earth elements. Understanding their structure provides a means of exploiting them.
- Fluorescence correlation spectroscopy using probes of varying sizes fixed the lower limit of the size of the two domains.
- Single-molecule tracking experiments reveal two domains between 20 and 50℃. Above 50℃, there is only one.
- Computational studies are consistent with the nanodomains arising from a first-order, liquid-liquid phase transition.
Opare-Addo, J et al., Nanodomains and Their Temperature Dependence. J. Phys. Chem. B. https://doi.org/10.1021/acs.jpcb.4c05184
Maddala, B.G. et al., Evidence for Nanostructures of at Least 20 nm. J. Phys. Chem. B. 2024, 128, 11197-11207. https://doi.org/10.1021/acs.jpcb.4c04950
V. Nguyen; et al., Automated Characterization of Spatial and Dynamical Heterogeneity in Supercooled Liquids via Implementation of Machine Learning. J. Phys. Condens. Matter 2023, 35, 465401. https://doi.org/10.1088/1361-648x/acecef
