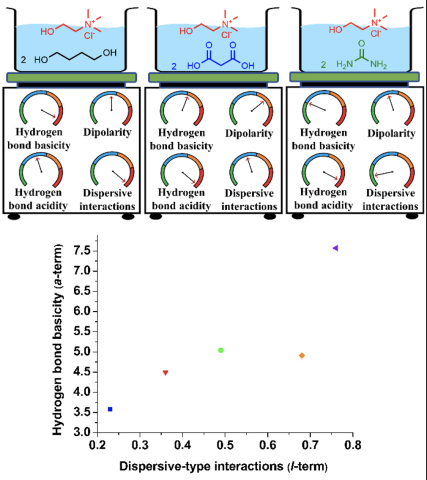
Solvation properties of choline chloride-based deep eutectic solvents (DESs) were thoroughly characterized using gas chromatography for the first time. Solvation models were constructed for various DESs to describe their individual solvation interactions and used to interpret and explain DES behavior in previously reported catalysis, separations, and organic synthesis studies.
The models developed in this study enable us to predict the performance of DESs in catalysis and separation systems based on their solvation characteristics. This advancement provides a framework for the selection of optimal DESs in various applications that could result in significant cost savings in energy-intensive separation processes.
- Choline salts form DESs with isomers of butane diol and hexane diol that possess higher hydrogen bond basicity and dispersive-type interactions compared to those composed of urea, acetamide, and organic acids.
- Employing organic acids as hydrogen bond donor significantly enhances the hydrogen bond acidity of choline chloride ([Ch+][Cl-]) DESs.
- Substituting chloride anion with acetate in choline salts decreases dipolar interactions while enhancing the hydrogen bond basicity.
- Employing different structural isomers of butane diol as hydrogen bond donor can significantly influence hydrogen bonding interactions.
Abbasi, N.M., Farooq, M.Q., Anderson, J.L. “Investigating the Variation in Solvation Interactions of Choline Chloride-Based Deep Eutectic Solvents Formed Using Different Hydrogen Bond Donors.” ACS Sustainable Chem. Eng. 2021, 9, 35, 11970-11980.
