CMI researchers from Oak Ridge National Laboratory conducted the research for this highlight.
Innovation
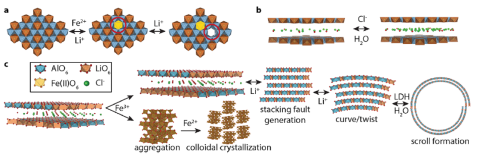
Fe-doped layered double-hydroxide (Fe-LDH) sorbents improve lithium extraction from brines.
Achievement
Cryogenic electron microscopy revealed the structure variability of Fe-LDH phases and reactions during sorption. Composition and hydration enthalpy depend on sorbent size and morphology.
Significance and Impact
- New understanding enables improvements in implementing the Fe-LDH sorbent for lithium recovery.
- Next steps entail partnership with industry for scale-up and demonstration under external funding.
Hub Targets Addressed
Unlocking unconventional resources. Highly selective separation from complex sources.
Citation: M. L. Whittaker, W. Dong, K. Li, T. Aytug, S. F. Evans, H. M. Meyer III, B. A. Moyer, and M. P. Paranthaman, “Cooperative Lithium Sorption in Doped Layered Double Hydroxides Is Modulated by Colloidal (Dis)Assembly” Chem. Mater. (2023). https://doi.org/10.1021/acs.chemmater.3c00072
