CMI researchers at Idaho National Laboratory, Garrison Minerals and Irish Metals conducted the research for this highlight
Innovation
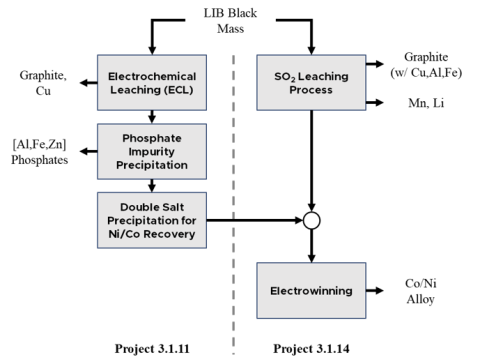
Co + Ni from LIB black mass recovered using electrowinning (EW) after electrochemical leaching, impurity removal by phosphate precipitation, and Tutton salt formation.
Achievement
Co/Ni rounds with a purity of +99.5% were recovered from LIB black mass. EW was shown to be effective for producing Co/Ni metal from a leachate without using conventional Solvent Extraction (SX) or Ion Exchange (IX) methods for purification prior to metallization.
Significance and Impact
Using SX and IX followed by crystallization or EW is the traditional way to produce high purity salts or metal products. Combining the EC leach, phosphate impurity removal, and Co/Ni double salt recovery steps with EW can produce high purity metal product from LIB black mass at potentially much lower environmental, capital, and operating cost.
Hub Goal Addressed
Advancing environmentally friendly and efficient recovery of critical materials from end‐of‐life energy storage devices.