CMI researchers at industry partner Terves, Ames Laboratory, Oak Ridge National Laboratory and Worchester Polytechnic Institute (WPI) conducted the research for this highlight
Innovation
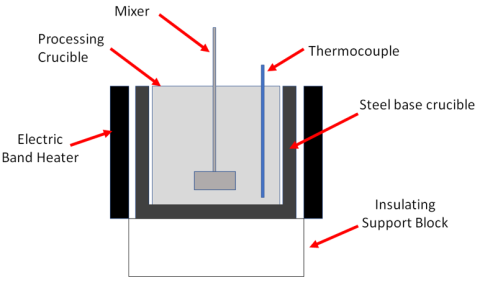
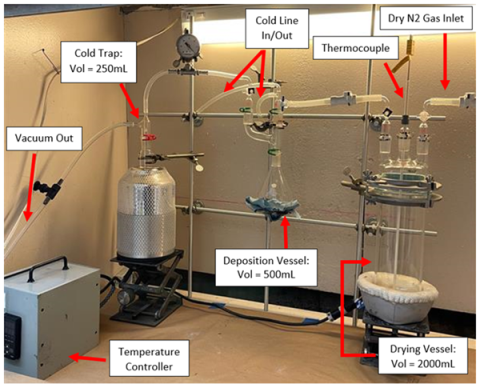
Development of a dry chloride (NdCl3) production and handling method through creation of solid ingot that can be fed directly into a reduction reaction under molten salt, producing a eutectic alloy (Fe or Mg).
Achievements
Developed a process to convert Nd2O3 to hydrated NdCl3 and established chloride drying and reduction reactors to be used to make Nd and Nd alloyed metal for purification at Ames Laboratory.
Significance and Impact
The reduction process can be run in a semi-continuous mode via removal of the alloy and/or salt throughout the process. Establishes a domestic producer of rare earth metals and alloy.
Hub Goal Addressed
Creating a domestic source of rare earth element production.